Want to go green and circular? Try Microalgae!

Savage Algae
Living in Finland, visiting the Mediterranean feels like suddenly bathing in heat and dazzling sunlight – especially directly after a snowy Vappu. I can’t help but to feel overwhelmed with the buzzle of life, colors, sounds and smells in the streets of Lisbon, balancing my heavy backpack, handbag and camera while trying not to crush the conference poster under my arm. I will need it to present my research at the Young Algeneers Symposium, taking place in Faro, another 4h drive towards the very South of Portugal.
While the conference participants are fighting against getting too drowsy in the heat, or study subjects – seaweeds, microalgae and cyanobacteria – are loving these conditions and are working overtime in the nearby facilities of the company Necton, which we had the chance to visit. Here, microalgae grow in long tubular bioreactors, the oldest of their kind in Europe, operating since 1999; until they are harvested to be turned into feed, food and cosmetics. Despite the success of the company, demonstrating the feasibility of mass microalgae production, a comment of João Navalho, president at Necton, stuck with me: Harvesting these microalgae may be like picking the weeds in a rich orchard – as far more productive microalgae species may still be out there, undiscovered (200,000-800,000 species are estimated and only 50,000 described!). The same goes for cyanobacteria, the other class of photosynthetic microorganisms (3,000 – 6,000 species estimated and 2,600 already described1). Moreover, the microalgae species we are already using are still undomesticated, savage, very much like those wheat stalks humans first gathered 11,000 years ago, holding only a few meager grains. Only after centuries of human selection they became capable of feeding the world with fat rows of grains on each stalk. Given the rapidly developing challenges we face today like climate change and world hunger – could we be a bit faster with the microalgae?
Taming Algae

The variety of applications covered during the three days of conference was amazing – bioremediation, wastewater treatment, production of food, feed, medicine, pigments – algae and seaweed seem to be able to do it all. What is more, their productivity is contagious – time to get back to the lab! I do so with a refreshed sense of motivation – microalgae and cyanobacteria truly have the potential to be a vital building block for a Circular Bioeconomy. To get there however, we need to keep searching for novel (micro)algae strains with new capabilities as well as develop our current production strains into better and better crop. And to do so, for the first time in history, we can fast-forward strain domestication via randomly creating mutants and selecting for those with high accumulation of the desired product (e.g. a pigment). We can even think outside of the evolutionary box and e.g. delete certain genes while introducing new ones from entirely different species. Through this genetic engineering we can enhance the efficiency with which the cells convert sunlight into energy and/or the desired product e.g. biofuels (like hydrogen) or fine chemicals (like building blocks for plastics). Thus, with the ever increasing knowledge of photosynthesis and the rapidly developing tools of genetic engineering, we are entering a new era of domestication – in which microalgae and cyanobacteria, due to their (relatively) easy accessibility towards genetic engineering, high photosynthetic efficiency as well as fast and easy cultivation, will surely play an important role.
Finnish Algae
Countless research projects are helping to pave the way towards microalgae based biotechnological applications – also here in Finland. I am working in the Photomicrobes group from the University of Turku, which is the only group in Finland studying the molecular mechanisms of photosynthesis, while also exploring possible biotechnological applications in different areas, from ethylene and hydrogen production to wastewater treatment, biopesticide and biofertilizer applications and genetic engineering to enhance the efficiency of light energy to product conversion.
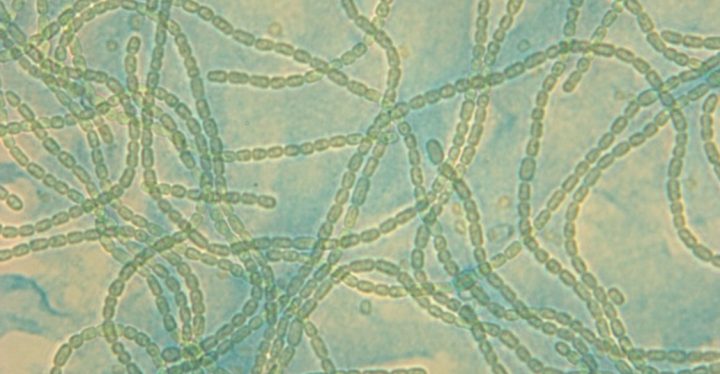
This is also where my PhD project comes in. I am working towards bioengineering Anabaena PCC7120, a filamentous freshwater cyanobacterium. It consists of different cell types that could potentially be used to produce different products concomitantly, for examples fine chemicals and hydrogen. In the future we could, next to growth in photobioreactors like in Faro, even trap bioengineered Anabaena (and also other species like the green algae Chlamydomonas) in hydrogels, fitting them into flat panels which could be easily installed e.g. on buildings or, considering Finland’s cold climate, in greenhouses, cleansing air and water from pollutants while converting the suns energy into useful products, creating a circular and literally green bioeconomy.
Elisa Werner
The author is doing her PhD in the Photomicrobes group – an interdisciplinary group working on the utilisation of microalgae and cyanobacteria in biotechnological applications. She is interested in the cyanobacterium Anabaena PCC7120, investigating strategies for strain engineering.
Main image: Microscopic image of the filamentous cyanobacterium Anabaena PCC7120.
- NABOUT, Joao Carlos, et al. How many species of Cyanobacteria are there? Using a discovery curve to predict the species number. Biodiversity and conservation, 2013, 22. Jg., S. 2907-2918.