The author of this post is Barbara Class, a PhD student at the section of Ecology of UTU working on evolutionary quantitative genetics of animal personality (See description on the VERG page). On her free time Barbara likes reading books, hiking, baking and watching funny (cat) videos.
It is now well known that the world’s biodiversity is declining at unprecedented rates and organisms are facing rapid changes in the biotic and abiotic conditions to which they are currently adapted. In this context, they only have two alternatives: to adapt or to die. Luckily, there is growing evidence that evolution is occurring on a contemporary time scale and has more importance for the species persistence on the short term than previously thought (Kinnison et al. 2007, Stockwell et al. 2003). This rapid evolution motivated an increased focus on the adaptive responses to environmental changes, a field that situates at the intersection of evolution, ecology and demography (Ferriere and Legendre 2012, Bell 2013, Vander Wal et al. 2013). This is because the adaptation of individuals will determine whether their populations will persist or not after rapid environmental changes. Particularly, a phenomenon of adaptive response to environmental changes observed at the population level has recently raised the attention of evolutionary biologists and conservationists: the evolutionary rescue.
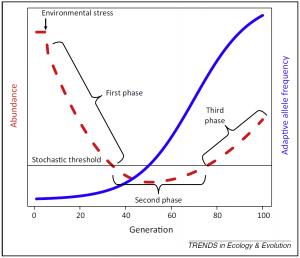
The main feature of evolutionary rescue is a U-shaped demographic time series showing a first phase of population decline followed by stabilization and recovery, parallel to an increase in the frequency of an adaptive phenotype (Figure 1, Carlson et al. 2014). An abrupt change in environmental conditions will often generate maladaptation of the population which was adapted to an initial set of environmental conditions. This means that the environmental change generates a difference between the mean population phenotype and the optimal phenotype. Consequently, the fitness (survival and/or reproductive success) of the most common phenotypes will decrease and cause the population’s decline. During the phase of stabilization, the population size falls below a stochastic threshold during which it is more susceptible to extinction due to random fluctuations. If the population size does not stay too long under this threshold, some individuals that express phenotypes closer to the new optimum (appeared due to de novo mutations or already present due to standing genetic variation), will have a better fitness than the others and produce more offspring. As a result, this will increase the proportion of better adapted individuals in the population and allow the population to recover (Gomulkiewicz and Holt 1995). Parallel to this demographic pattern is an increase of the frequency of adaptive alleles which determine phenotypes that are beneficial under the new environmental conditions.
The concept of evolutionary rescue is not new (this is what is causing antibiotics resistance for instance) but has the potential to be used as a tool for the conservation of species, provided we understand the processes underlying it. We could for example predict which species would be able to cope with environmental changes for certain timescales and take conservation measures to promote the evolutionary rescue of endangered species.
To date, evolutionary rescue has been mostly studied in the lab where many environmental factors can be controlled and in species that are in general small, short-lived and easy to maintain in large populations in laboratory conditions. In contrast, evidence for this in wild populations is still relatively scarce. This reflects the logistical challenge of monitoring wild populations over long periods of time to get demographic and evolutionary data in addition to measuring all the environmental factors that can influence their persistence (Gomulkiewicz and Shaw 2013).
A recent paper published in Nature Communications might add to the few examples of evolutionary rescue occurring in the wild. The Tasmanian devil’s facial tumour disease (DFTD), discovered in 1996 has been responsible for the Tasmanian devil’s (Sarcophilus harrisii) severe population decline (on average 80%) over the last two decades. DFTD is a highly contagious cancer transmitted through biting during social interactions which has been shown to hide from the host’s immune system (Siddle et al. 2013) and to provoke its death within 6 months. Although the situation seemed hopeless for the species, a team of scientists started to investigate why some populations which were predicted to be already extinct persisted in low numbers. The comparison of DNA collected from 3 populations before and after the disease occurred attributed this persistence to two genomic regions containing genes related to immune function and cancer in other mammals. The frequency of some alleles in these two regions changed independently in the 3 populations in just a few generations and these alleles provided an increased fitness for individuals carrying them. This suggests that the Tasmanian devil is evolving a resistance mechanism against this tumour and that there might be some hope for the future of the species. For instance the favourable alleles could be increased in the population by a breeding program incorporating only resistant individuals. However, it may also have drawbacks: evolutionary rescue might help the Tasmanian devil to resist to its fatal tumour but can also make it more vulnerable to other threats due to the depletion of genetic variability of the population. Indeed, a second transmissible tumour (DFT2) appeared recently and provokes similar symptoms as DFTD but it is not known yet whether the recently evolved resistance mechanism will help the Tasmanian devil fight this new disease.
The article in question:
Epstein B, Jones M, Hamede R, et al (2016) Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat Commun 7. doi: 10.1038/ncomms12684
Other references:
Bell G (2013) Evolutionary rescue and the limits of adaptation. Philos Trans R Soc Lond B Biol Sci 368:20120080. doi: 10.1098/rstb.2012.0080
Carlson SM, Cunningham CJ, Westley PAH (2014) Evolutionary rescue in a changing world. Trends Ecol Evol 29:521–530. doi: 10.1016/j.tree.2014.06.005
Ferriere R, Legendre S (2013) Eco-evolutionary feedbacks, adaptive dynamics and evolutionary rescue theory. Philos Trans R Soc Lond B Biol Sci 368:20120081. doi: 10.1098/rstb.2012.0081
Gomulkiewicz R, Holt RD (1995) When does Evolution by Natural Selection Prevent Extinction ? Evolution (N Y) 49:201–207.
Gomulkiewicz R, Shaw RG (2013) Evolutionary rescue beyond the models. Philos Trans R Soc Lond B Biol Sci 368:20120093. doi: 10.1098/rstb.2012.0093
Kinnison MT, Hendry AP, Stockwell C a. (2007) Contemporary evolution meets conservation biology II: Impediments to integration and application. Ecol Res 22:947–954. doi: 10.1007/s11284-007-0416-6
Siddle H V, Kreiss A, Tovar C, et al (2013) Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proc Natl Acad Sci U S A 110:5103–5108. doi: 10.1073/pnas.1219920110
Stockwell CA, Hendry AP, Kinnison MT (2003) Contemporary evolution meets conservation biology. Trends Ecol Evol 18:94–101. doi: 10.1016/S0169-5347(02)00044-7
Vander Wal E, Garant D, Festa-Bianchet M, Pelletier F (2013) Evolutionary rescue in vertebrates: evidence, applications and uncertainty. Philos Trans R Soc Lond B Biol Sci 368:20120090. doi: 10.1098/rstb.2012.009